- europages
- >
- Medical Equipment
- >
- EC PLAZA
- >
- EarlyTect® Colon Cancer
EarlyTect® Colon Cancer
Description
Product Description EarlyTect Colon Cancer Test was approved by the Korea Ministry of Food and Drug Safety through clinical trials at Yonsei Medical Center, Severance Hospital, and Severance Check-up Center for eligible subjects, 585 men and women aged 30 to 80. Clinical trials using fecal DNA demonstrated that CRC can be diagnosed with 90% sensitivity and specificity. EarlyTect Colon Cancer Test is a qualitative real-time PCR test that measures methylated Syndecan-2, a biomarker clinically related to precancerous lesions of CRC, in stool DNA to diagnose colorectal cancer. This product is not intended to confirm colorectal cancer, so if a subject was positive, CRC or precancerous lesions of CRC may exist. Product Standard - Cancer Type: Colorectal Cancer - Subject: Subjects for asymptomatic colorectal cancer screening - Biomarker: SDC2 me The price and transaction conditions are negotiable. Please Request a Quote for the transaction
- Medical Equipment
- cancer test
- Colorectal cancer test
- Colorectal cancer screening
Similar products from EC PLAZA
EC PLAZA
South Korea
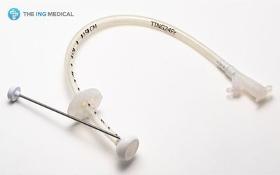
1) Product Description The Gastrostomy Feeding Tube are indicated for use in patients who require long term feeding, are unable to tolerate oral feeding, who are at low risk for aspration, require gastric decomparession. It is a tube used to supply food and drugs to the stomach. 2) Product Features - Easy and less traumatic to insert due to the soft and compressible internal dome. - Radiopaque dome allows x-ray placement confirmation - Identification of tube obstructions enhanced with translucent tubings - Soft, less traumatic silicone designed for patient comfort
Request for a quote
EC PLAZA
South Korea
Negative Pressure Carrier is used for isolating confirmed or suspected cases of infection from medical staff or paramedics. Our product is not only easy to carry but also able to take CT scans because of its air frame structure. It is equipped with an apparatus for maintaining negative pressure inside of a carrier, and HEPA filter for filtering hazardous pathogens. There is also auxiliary battery for emergency case besides embedded battery. 1. Certified for Korean GMP,CE,ISO 13485 2. Prevent secondary infection after filtering high risk pathogens. 3. Detachable from a stretcher. 4. Possible to do CT scans. 5. Simple installation in less than 5 minutes. 6. Easy to carry because of light weight(10kg) 7. Competitive price
Request for a quote
EC PLAZA
South Korea
IWIN is a company that makes AUTOZEN,an equipment that produces slightly acidic hypochlorite acid water, one of the sterilized water. Slightly acidic hypochlorite acid water is gradually expanding its scope of use due to food hygiene management, prevention and sterilization of agricultural and livestock diseases, and prevention of infection in hospitals. AUTOZEN is a slightly acidic hypochlorite acid water manufacturing device approved as a food additive and provides a total solution for eco-friendly sterilization, deodorization, and disinfection.
Request for a quote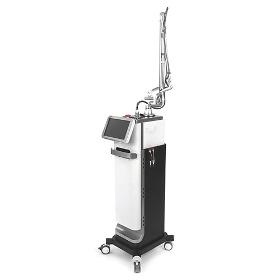
EC PLAZA
South Korea
BIOXEL uses fine laser beam to minimize skin damage by heat and to shorten the recovery and rejuvenation period. Treatment time can be shortened by adjusting spot size and the beam density, which is beneficial for both of the operator and the patient. [Features] Fractional Mode & General CO2 Mode & Dental Mode & LVR Mode Reduced Pain & Excellent Effectiveness Three Patterns : ARRAY, GRID, RANDOM Smart & Various Protocols for User’s Convenience Max 4489 dots to an area of 20x20mm Various scan shapes: Square, Hexagon, Triangular, Circular [Application] Fractional Hand Piece Skin tightening, Wrinkles, Large Pore, Skin Resurfacing, Acne scar, Stretch Mark, Laser Peeling, Age Spot, Pudenda, etc. General Hand piece (50mm or 100mm) Mole, Wart, Freckles, Birthmarks, Tumor elimination, Condylom, Syringoma, Snoring.
Request for a quote