- europages
- >
- Import-export - medical and surgical equipment
- >
- MAXCERT LTD
- >
- Person Responsible for Regulatory Compliance (PRRC)
Person Responsible for Regulatory Compliance (PRRC)

Description
The new European Commission’s guidance on persons responsible for regulatory compliance (PRRC) explains that manufacturers, Authorized Representatives and micro and small manufacturers must designate at least one staff member responsible for ensuring compliance to the MDR and/or IVDR as appropriate according to Article 15 of the Regulation. PRRC qualifications are specific for each of these three operators. This does not mean that you have to hire PRRC as employee rather you can avail our PRRC service to overcome the regualtory needs of your company and medical devices – with a consultant managing the system on a few days per month basis. We would be more than happy to discuss this option with you, helping you to find a consultant with the necessary qualifications to perform this service effectively. If you would like to know more about this service, then please contact us.
- Import-export - medical and surgical equipment
- PRRC
- UKRP
- EUMDR
Similar products
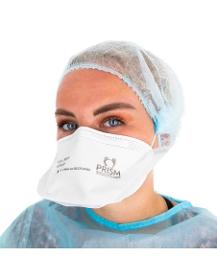
PRISM
France
• FFP2 half mask, non-reusable (NR), duckbill type. • Total back length: 225 mm= 8,85827 in, small size. • This FFP2 mask for personal respiratory protection is single use & non-sterile. • PRISM masks are manufactured on our production line in Frontignan in the Hérault region of France & meet the recommended European and French standards. • Double certification : - NF EN 149+A1 2009 ; - 14683 + AC 2019. • PRISM carries out quality controls as it produces. • COMPOSITION 1/ A "duckbill" mask organised in several layers: - the outer layers in non-woven polypropylene (= spunbond) ; - the two intermediate layers ensure filtration with meltblown fabric. 2/ A double nose bar to adjust the mask to the shape of the nose & to reduce fogging of the glasses. 3/ A double flat elastic band (manual separation) to secure the mask to the face.
Request for a quote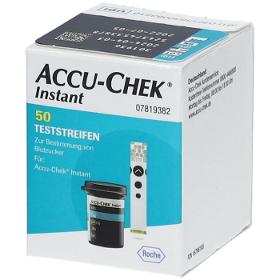
COMFORTPAT B.V.
Netherlands
Accu Chek Instant test strips For determining blood sugar Suitable for self-application Suitable for the following blood glucose meters: Accu-Check® Instant Accu-Check® Instant S
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
Stent manufacturing applies to a wide variety of medical applications including cardiovascular applications, birth control, kidney stone pain control and esophageal and gastrointestinal uses.
Request for a quote
TEMAS GROUP EXPORT PARTNERS
Turkey
MUSTANOGLU PROFESSIONAL MEDICAL EQUIPMENTS SALON DEVICESREGIONAL REVIEW REGIONAL THINNING DIODE LASER EMS SLIM EMS HIEMT 7D HIFU 4D HIFU HYDRAPRO Q-SWITCH PLASMA G5 LENF DRAINAGE INBODY PDT SKIN ANALYSIS ROLL SLIM
Request for a quoteRequest for quotes
Create one request and get multiple quotes form verified suppliers.
- Only relevant suppliers
- Data privacy compliant
- 100% free