
You can visit us at Medlab Hall 24 Stand J29
Turkey
Manufacturer/ Producer

DISERA MEDICAL EQUIPEMENT
Turkey
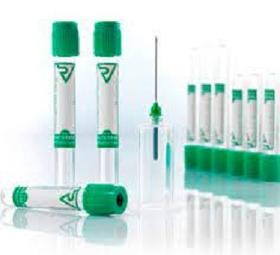
Vacusera Heparin Tubes are coated with Lithium Heparin or Sodium Heparin to inhibit clotting. Sodium Heparin or Lithium Heparin Tubes are used for plasma determinations in clinical chemistry. Lithium Heparin Tubes are also available with gel separator.
Request for a quote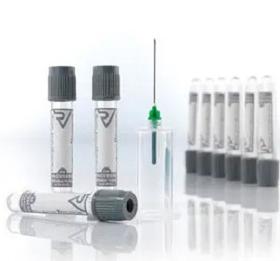
DISERA MEDICAL EQUIPEMENT
Turkey
Vacusera Glucose Tubes contain with Sodium Fluoride and K3 EDTA. Glucose tubes are used for glucose determinations like blood sugar, sugar tolerance.
Request for a quote
DISERA MEDICAL EQUIPEMENT
Turkey
100 ml. volume Graduated Writing surface Leak proof Screw Cap 100,000 class cleanroom production Manufactured according to the 98/79/EC In Vitro Diagnostic Directive
Request for a quote
DISERA MEDICAL EQUIPEMENT
Turkey
100 ml. volume Graduated Writing surface Leak proof Screw Cap 100,000 class cleanroom production Manufactured according to the 98/79/EC In Vitro Diagnostic Directive
Request for a quoteOur production and sales activities have expanded since DISERA's inception in 1996. The product portfolio for the 2004 investment included Petri dishes, sharp boxes, vaginal speculums, and urine beakers. Our production investment reached a new level in 2014 as a result of the production of evacuated vacuum tubes. VACUSERA vacuum blood collection tubes are the result of advanced manufacturing technology and diagnostic experience. We moved to a new factory building in Turkey's Tire Organized Industrial Area in 2014. The entire manufacturing process adheres to ISO 13485: 2016 quality management systems, and all products bear the CE mark. We provide manufacturing services in accordance with ISO 13485: 2016 quality standards and have knowledge and experience in providing high-quality, technical medical products that meet the needs of the healthcare sector. Vacusera Blood Collection Tubes, for example, are CE marked in accordance with Directive 98/79/EC on In Vitro Diagnostic Devices. Vacusera Blood Collection Tubes are manufactured in accordance with ISO 6710 and EN 14820 standards. Furthermore, Vacusera Blood Collection Needles are CE certified and manufactured in accordance with 93/42/EEC regulations. To increase our production volumes, we invest in new facilities. We are aware of the current global market demand for laboratory consumables. Furthermore, this demand will grow in the future.

You can visit us at Medlab Hall 24 Stand J29
Manufacturer/ Producer
Karabaglar mahallesi, 5758. Sk. 4 H D:11
35110 Izmir - Turkey