- europages
- >
- Biotechnologies
- >
- EOSWISS PHARMA
- >
- products
EOSWISS PHARMA - Your partner for technology and production transfer in the pharmaceutical industry
Switzerland
Service Provider


EOSWISS PHARMA
Switzerland
We manage investment projects for you, such as production expansions, installation of new plants, greenfield construction projects, brownfield construction projects. We focus on the rapid implementation of your project. With these short project durations, you have low project costs and a high return on investment. We adapt our project management to the very specific circumstances of our clients Contact us for an initial discussion! This makes our project management flexible enough to handle the changes that occur in every project. Our focus is on short "time to market" and thus low project costs. This is the only way for projects to pay off quickly. We execute projects very quickly and achieve significant time savings compared to traditional project management. Pharmaceutical production •After qualification and commissioning, we also take care of the start of production. •Optimisation of the production parameters •Validation •Transfer to routine operation
Request for a quote
EOSWISS PHARMA
Switzerland
We carry out projects quickly and without loss of quality. You have short project durations, thus lower costs and a quick return on investment. Contact us for an initial discussion! Our projects often combine Technical and regulatory issues: •Technology & technology transfer (adapt, improve technologies) •Optimise in production •Quality projects (Quality by Design "QbD", Process Analytical Technology "PAT", test design, validations, qualifications) •Short processing times in administration •Improve the supply chain We optimise supply chains, develop suppliers, um/damit: •"Shorten "Lead Times •the supplier reacts quickly •broaden the supplier base •find new suppliers •Relocate productions We take over technical projects like: •"Scale up" in biotechnology and pharmaceuticals •Model and simulate production steps in biotechnology and pharmaceuticals •Improve production processes (quality, process) •modelling new or modified buildings ("virtual factory") •simulate
Request for a quote
EOSWISS PHARMA
Switzerland
Freeze-drying is an important process for preserving and formulating pharmaceutical and biotechnological active ingredients. If an existing process is changed or transferred, the entire cycle must be modified. We advise on the adaptation of a freeze-drying cycle or scale up. Contact us for an initial discussion! Analysis We analyse existing data, model the process and verify the settings before the conversion. For them, this means: high transfer security, short project times, low overall costs. Adaptation of a freeze drying How do we go about it? For the time being, we analyse all existing data and "build" a model of freeze drying from it. The model is based on the physical principles and the real data of their current plant. Consequently, our predictions and forecasts are accurate. From the model, we obtain the settings for the new freeze-drying process. Before the step into production, these settings are verified with a small system.
Request for a quote
EOSWISS PHARMA
Switzerland
Statistical experimental design We plan your trials in development and production. With our statistical methods, you can optimise production during normal operation. This saves you technical batches and thus costs. Nevertheless, you gain resilient and robust improvements. Statistical design of experiments is the fastest and most cost-effective way to carry out developments and optimisations. Contact us for an initial discussion! CPV "Continouus Process Verification With CPV, the manufacturing process is checked for variable proportions, trends and opportunities for improvement. As such, it is part of the "Life Cycle Management" to further operate the process in a controlled manner within narrow limits. We evaluate your process data statistically and thus show potential for improvement. This way you use your data productively and can achieve strong savings and improvements in quality. So it pays off! Contact us for an initial discussion! Quality by Design "QbD
Request for a quote
EOSWISS PHARMA
Switzerland
The step from the laboratory to manufacturing for clinical trials is a major challenge. Simple hand movements in the laboratory often cannot be easily translated into process steps of industrial manufacturing. We are specialised in such scale ups. Contact us for an initial discussion! Analysis We prepare your laboratory data and compare it with the possible process parameters at the contract manufacturer. If data or optimisations are still missing. We develop the test plan for this. We carry out certain scale-up trials (non GMP) at our technical centre. We accompany specific trials (e.g. with your API) on your premises or those of a plant manufacturer. Models and simulation From the data, we generate the process models you need for scaling up. You will also need these models for the submission documents. We use the data to simulate different scale-up concepts. So if you later transfer from the special CDMO for clinical production to the CDMO for commercial production
Request for a quote
EOSWISS PHARMA
Switzerland
Based on your requirements, we search for and find suitable contract manufacturers. We prepare your tender documents and make a pre-selection of suitable contract manufacturers (CMOs, CDMOs). Contact us for an initial discussion! We evaluate the CMOs for this in a structured scheme. We use not only the bid price, but also the important parts of the bid: •Flexibility •Smallest order quantity ("MOQ") •Terms of payment •Logistics opportunities •Development support •Potential for the future This allows you to choose the best contract manufacturer for your project! You then transfer your technology or product, or hand over this work package to us. We transfer even complex productions and technologies within 6 -12 months. This means you are on the market very quickly, your resources are not blocked. Contact us for an initial discussion!
Request for a quote
EOSWISS PHARMA
Switzerland
The strong supply chain is the basic prerequisite for a strong brand, even for completely virtual pharmaceutical companies such as biotech start-ups. We search and find new contract manufacturers (CMO, CDMO) for you. Contact us for an initial discussion! You benefit from our proven evaluation system that we have developed for various industries. Because with the CMO, it's not just the price of 1 packaged unit that matters, but also the price of the product: •Flexibility of delivery dates and minimum order quantity •Terms of payment •Logistics solutions •Packaging option •Audit status •Development support •Potential of the contract manufacturer Depending on your individual requirements profile, we find suitable contract manufacturers with our carefully prepared tender documents. In this way, you reduce your overall costs and further develop your suppliers. Contact us for an initial discussion! Supplier management We take over the continuous control and improvement
Request for a quote
EOSWISS PHARMA
Switzerland
We carry out the entire technology transfer or production transfer from a manufacturer to a contract manufacturer (CMO, CDMO) for you. Contact us for an initial discussion! In the process, we look for the right CMO for you and prepare a pre-selection. You decide on the best manufacturer, then we carry out the entire transfer for you: •Planning resources •Timetable including work packages •Structure the cooperation with the CMO •Prepare all necessary documents •technical transfer •Adjusting the production parameters, optimisation •we validate the transferred production process •handed over to routine production We bring all the necessary specialists into the project ourselves, or work hand in hand with your internal team. Depending on how it is needed. We carry out transfers very quickly. This shortens the project duration and reduces your costs: They are quickly back on the market! This reduces transfer costs in the long term. Contact us for an initial discussion!
Request for a quote
EOSWISS PHARMA
Switzerland
We plan and perform your complete process validation. From the first gap analysis and risk management, planning, execution and reporting. We support you with full project management, providing support where needed. You free your ressources for your projects, we take care for validations and all connected activities. You reach your goals safely, quicker and faster.
Request for a quote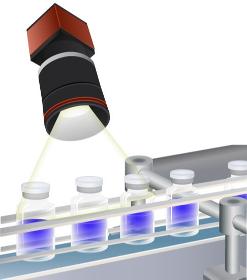
EOSWISS PHARMA
Switzerland
We support your manufacturing with our polarised light camera and analytical software. We film production issues of transparent material (vials, blisters, glass items, foils) with polarized light. Defects, scratches, flaws, residual tensions brought in during manufacturing become visible. Hence, they can be corrected and improved. You receive an improved manufacturing process, with higher quality and higher capacity.
Request for a quote