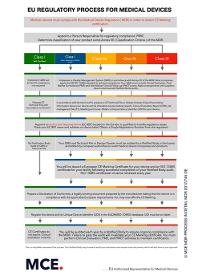
Maxcert can provide the strategic and technical guidance to develop an appropriate project plan, as well as assist with the implementation of any stage of the MDR certification process. QMS / Risk Management - Strategy for Regulatory Compliance - MDR-Specific QMS Changes - Classification & Conformity Assessment - Other EU MDR Directives & Regulations - Risk Management Evaluation Criteria - Benefit Risk Analysis - MDR Readiness Audits Clinical Evidence - Guidance on New Clinical Evidence Requirements - Legacy Product Data Review - Performance Evaluation Plan / Report (PEP/PER) - Analytical Performance Report - Clinical Performance Plan / Report - Clinical Performance Study Plan (CPSP) - Scientific Validity Report Labelling & UDI - Intended Purpose / Claim Review - Labels & Instructions for Use Requirements - Applicable Standards - UDI Requirements & Symbols Technical Documentation - Technical Documentation Review & Preparation - GSPR Gap Assessments & Traceability Matrices - Test, Hardware, Software Requirements Post-Market Surveillance / Vigilance - Post-Market Surveillance Plan / Report - Post-Market Follow-up (PMPF) Plan / Report - Incident Reporting - Periodic Safety Update Report (PSUR) Economic Operators / EUDAMED - Person Responsible for Regulatory Compliance (PRRC) - Registration of EO’s & Devices - Control of Suppliers (Process/Method) To speak to an MDR expert, or if you don’t see the services you are looking for, contact us