- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- surgical instruments
Results for
Surgical instruments - Import export

MAXCERT LTD
Germany
Maxcert is offering EC Representative services for non EU medical devices manufacturers. Maxcert is authorized/ registered EC REP service provider and can manage your ECREP requirements better than others. We make sure that you are MDR ready! and well updated with the EU regulations for your medical devices. Maxcert’s team has expert knowledge to help you navigate through the complex regulatory challenges that the new EU MDR bring. We can assist you through out the entire process to ensure that you and your business are compliant with all of the EU MDR requirements. For more information, reach out to us at
Request for a quote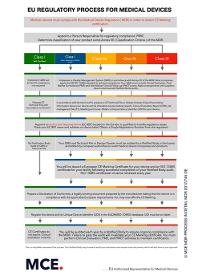
MAXCERT LTD
Germany
Our experienced team has for years done documentation for companies who are manufacturers of medical devices /surgical instruments. We provide Quality-Management-Systems for meeting legal and normative requirements. For manufacturers of medical devices / surgical instruments class I (including Is, Im, and new for MDR class Ir), IIa, and IIb we offer the following services: Implementation DIN EN ISO 13485 and the EU-Regulation 2017/745 MDR and 21 CFR 820 QSR Creating QM-Systems with procedural instructions and work instructions Technical Documentation Clinical evaluation Risk Management / Risk Analysis Essential Safety and Performance Requirements Validation of processes UDI-labeling Consulting for Regulatory Affairs Internal audits and external supplier audits Accompaniment and support with inspections by authorities and Audits by notified bodies Communication with authorities
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
Results for
Surgical instruments - Import exportNumber of results
2 ProductsCompany type