- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- medical devices
Results for
Medical devices - Import export

AMETEK ENGINEERED MEDICAL COMPONENTS
United States
The DENSYTY® Interconnect System uses proven card edge technology to maximize contact density, performance and usability. The need for high contact density in a small form factor is increasing.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK Engineered Medical Components (EMC) is heavily invested in cable assembly work for the patient monitoring device market. Cable assembly normally consists of multiple wires and connectors that are assembled into a single cable. The cable must give end-to-end connectivity while being flexible and durable enough to withstand daily usage.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
As a leading manufacturer for a wide range of medical device components, AMETEK EMC is at the forefront of this medical technology-focused field working with internal experts, engineers, and researchers to design, develop and refine technologies that ensure neuromodulation device components.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK Engineered Medical Components (EMC) specializes in manufacturing Electrophysiology (EP) Medical Devices as one of many key medical device segments. Design and manufacturing solutions
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK Engineered Medical manufactures advanced technology sensor components for different types of continuous glucose monitoring devices for its customers.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
Both medical and surgical equipment play a pivotal role in modern healthcare, enhancing the accuracy of medical procedures, ensuring patient safety, and contributing to improved clinical outcomes. Their continuous innovation and adherence to strict quality standards are vital to advancing medical practice.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK EMC has been a leading manufacturing partner for vascular devices for decades. In addition to engineering and manufacturing advanced vascular device components, we also help to improve existing medical devices in a wide variety of vascular projects.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK EMC has been meeting the emerging demand for minimally invasive structural heart procedures and critical cardiac components for several decades. Precision Nitinol components and welded assemblies along with complex delivery systems are some of the key products that AMETEK EMC manufactures for the structural heart market.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK EMC has been manufacturing in the orthopedic device market for decades. With regular technology improvements, we’ve adapted to the latest capabilities. Precision cutting of tube and sheet as well as precision welding with lasers is the key to our capabilities.
Request for a quote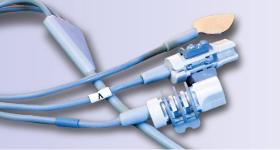
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
Silicone transfer molding is another manufacturing process used in the medical device industry. It is similar to LSR molding but focuses on more delicate electrical connections or instruments. It is a 2-step process that involves the manual molding of components into shape.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
There are multiple advantages that LSR offers over plastic injection molding to the medical device industry and in patient care.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
Laserage can precisely cut, drill, weld, scribe, and heat treat a wide variety of materials to meet any and all specification.
Request for a quote
UTAK
United States
For the purposes of drugs of abuse screening it is necessary to have a set of controls with high, intermediate and low drug concentrations. These cutoff controls allow identification of various levels of abuse.
Request for a quote
UTAK
United States
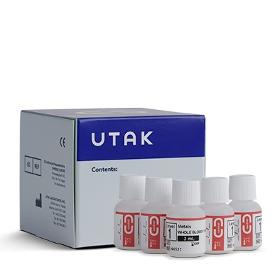
A validity control, which contains common adulterants, is utilized in drugs of abuse (DOA) testing to confirm a laboratory's DOA analytical systems are performing accurately and can identify adulterated urine specimens. Validity Controls Level 1 through 5 are used as quality control materials to help identify substituted, diluted, and adulterated urine samples. The matrices for this QC are prepared from distilled water. The levels of the Adulterants, Creatinine, Specific Gravity, and pH are adjusted to the desired range for each lot prepared. Quality control before, during, and after the preparation of the control material ensures that each lot is comparable and of the same high quality. The listed ranges were verified by analytical methods similar to those actually used in a testing laboratory.
Request for a quote
UTAK
United States
Our 100% Real Normal Human Serum is collected in FDA-licensed facilities within the United States from healthy adult donors. We screen our Normal Human Serum for common drugs of abuse and alcohol. Our frozen serum is suitable for all testing methods, including LCMS, LC-MS/MS, GCMS, Time of Flight (TOF), ELISA, EMIT and other immunoassays. This product uses Potassium Oxalate as an anticoagulant and Sodium Fluoride for preservation.
Request for a quote
UTAK
United States
Immunosuppressants are essential for successful organ transplants. Their drug concentrations must be routinely monitored to ensure optimal levels and patient compliance. Lower concentrations lead to transplant rejection; higher concentrations lead to severe adverse effects including Graft vs Host Disease.
Request for a quote
UTAK
United States
Anticonvulsant, or anti-epileptic drugs, are used to treat a wide range of symptoms affecting the nervous system. Most commonly they are utilized to control convulsive seizures, psychotic behavior and pain. Many of these drugs have very narrow therapeutic ranges and low thresholds for toxicity, meaning precision and accuracy are of the utmost importance. Our serum controls for anticonvulsants are an integral part of quality control for any lab that performs therapeutic drug monitoring.
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
UTAK
United States
Many toxicological specimens are whole blood. This control confirms a method's accuracy for quantitating heavy metals associated with poisoning in whole blood.
Request for a quote
UTAK
United States
Trace elements are essential compounds necessary for a wide variety biochemical reactions including oxidation-reduction, synthetic and metabolic processes. They are a required cofactor for many enzymes. Trace element deficiencies associated with a failure of those enzyme systems for which the element serves as a cofactor cause biological system failures and death. Trace element toxicity is associated with overdose, usually secondary to taking too much high dose trace element supplements.
Request for a quote
UTAK
United States
Our 100% Real Whole Blood is collected in FDA-licensed facilities within the United States from healthy adult donors. We screen our whole blood for common drugs of abuse and alcohol. Our frozen blank whole blood is suitable for all testing methods, including LCMS, LC-MS/MS, GCMS, Time of Flight (TOF), ELISA, EMIT and other immunoassays. This product uses Potassium Oxalate as an anticoagulant and Sodium Fluoride for preservation.
Request for a quote
UTAK
United States
Our 100% Real Human Urine is collected from healthy donors within the United States. We screen our urine for the following analytes: d-Amphetamine, d-Methamphetamine, Benzoylecgonine (BE), (-)-Cotinine, Morphine, Oxazepam, and (l)-9-Carboxy-11-nor-Delta-9-THC (THC-COOH). Our liquid urine contains Sodium Azide for preservation and is suitable for all testing methods, including LCMS, LC-MS/MS, GCMS, Time of Flight (TOF), ELISA, EMIT and other immunoassays.
Request for a quote
DALCO INTERNATIONAL, INC.
United States
Features a circumferential, semi-rigid shell that offers maximum support to the leg/ankle following injury or post-operative procedures. Available in a high boot (pictured) and low boot version. Product Features: Streamlined, semi-rigid shell is lightweight yet provides maximum support for the leg and ankle. Medial/lateral air bladders offer customized compression to increase comfort and reduce edema. Rocker sole helps promote a natural gait to allow continuation of daily activities. Shock-absorbing insole reduces impact of the heel strike while walking. Toe guard for added protection.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK EMC can provide processing of your implantable medical device components from laser cutting to the finished component.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
With multiple facilities worldwide, AMETEK EMC manufactures state-of-the-art medical device components and assemblies on a global scale.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
TSE’s design team creates custom connectors incorporating: component design and material selection, design for manufacturing (DFM) technique.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
AMETEK EMC offers a wide variety of laser welding capabilities for metal implant materials for the orthopedic industry.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
Laserage is widely regarded as the medical industry-leading expert in laser contract manufacturing. Since 1979, we have been leading the way in the field of custom laser processing, providing medical device component fabrication with numerous laser manufacturing capabilities.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
In-house 3D printing and a dedicated prototyping labs facilitate fully functional prototypes for quick assessments, precise design iterations, and shortens timing for design freeze. EMC’s experts can help customers select the right materials and assemble the right solution for the application’s requirements.
Request for a quote
AMETEK ENGINEERED MEDICAL COMPONENTS
United States
As a leading partner in the medical device market, AMETEK Engineered Medical Components (EMC) supplies customers in the electrosurgical instrument sector with critical, customized interconnect and cable solutions as well as precision laser-processed assemblies. This includes ENT (ear, nose, throat) and reconstructive surgery products
Request for a quoteResults for
Medical devices - Import exportNumber of results
43 ProductsCountries
Company type
Category
- Import-export - medical and surgical equipment (18)
- Biochemistry - products (10)
- Medical Equipment (9)
- Medical and surgical instruments (5)
- Steel & Metals (3)
- Lasers - medical applications (2)
- Laser - cutting and welding machines (1)
- Artificial pacemakers (1)
- Pipes and tubes, stainless steel (1)
- Prostheses, orthopedic (1)
- Silicones (1)
- Surgical implants (1)