- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- medical device
Results for
Medical device - Import export
TEMAS GROUP EXPORT PARTNERS
Turkey
MUSTANOGLU PROFESSIONAL MEDICAL EQUIPMENTS SALON DEVICESREGIONAL REVIEW REGIONAL THINNING DIODE LASER EMS SLIM EMS HIEMT 7D HIFU 4D HIFU HYDRAPRO Q-SWITCH PLASMA G5 LENF DRAINAGE INBODY PDT SKIN ANALYSIS ROLL SLIM
Request for a quote
FLYNTHER BV
Netherlands
•21 gauge lancet 2.0mm •Suggested test areas e.g. cholesterol, Haemoglobin (Hgb), diagnostic kit tests such as cardiac enzymes Side fire activation Unistik 3 puts the sampling experience firmly in your hands, with side-fire activation enabling you to decide exactly when to activate the device and how much pressure to apply. The alignment guide indicates precisely where the device will activate on the patient’s finger.
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Disinfectant solution for medical devices
Request for a quote
ALFA-MED LLC
Russia
The work of the TempoHeart module is based on the method of scoring the heart rate vegetative regulation. This method is popular due to its high information value and non-invasiveness. Cannot be used without an interface block. The module can: • evaluate the heart rate autonomic regulation; • to predict in advance (to prematurely reveal) the hazards of a myocardial infarction, hypertensive crises and strokes; • to determine the metabolic and electrolyte disorders in the myocardium; • identify risk groups for heart rate increased stability; • to make a cold evaluation of the autonomic nervous system's reaction when exposed to electromagnetic fields, intoxications and other pathogenic factors; • to assess the activity of the respiratory component when measuring heart rate variability, recording the parameters of blood movement in the vessels; • to expose the stress resistance level.
Request for a quote
DALCO INTERNATIONAL, INC.
United States
Features a circumferential, semi-rigid shell that offers maximum support to the leg/ankle following injury or post-operative procedures. Available in a high boot (pictured) and low boot version. Product Features: Streamlined, semi-rigid shell is lightweight yet provides maximum support for the leg and ankle. Medial/lateral air bladders offer customized compression to increase comfort and reduce edema. Rocker sole helps promote a natural gait to allow continuation of daily activities. Shock-absorbing insole reduces impact of the heel strike while walking. Toe guard for added protection.
Request for a quote
DMR MEDICAL
Turkey
We are able to supple all kind of medical products, however our main products lines are diabetic cares, dermal filler and laboratory reagents.
Request for a quote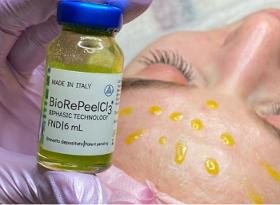
RAFI POLAND
Poland
BioRePeelCI3 FND is an innovative biphasic, medical device with the biostimulating, revitalising and peeling actions indicated for the face, neck, decolletage and the delicate intimate area with a 35% concentration of TCA as the main ingredient.

E-FILLERS.COM
Greece
HYAcorp Body Contouring MLF2© is an absorbable skin implant with a high level of purity. It is a medical device intended for single use only and is produced from a hyaluronic acid of non-animal origin. The special composition of HYAcorp Body Contouring MLF2© provides maximal volumizing results in body contouring.
Request for a quote
E-FILLERS.COM
Greece
ALIAXIN® GP (Global Performance) is a resorbable medical device to be used as a filler for the correction of medium and deep facial cutaneous sagging and to increase the volume and contours of the lips. The principal component is cross-linked Hyaluronic Acid of non-animal origin, produced by bacterial fermentation.
Request for a quote
E-FILLERS.COM
Greece
HYAcorp FINE© is an absorbable skin implant with a high level of purity. It is a medical device intended for single use only and is produced from a hyaluronic acid of non-animal origin. HYAcorp FINE© contains natural HA to moisturize the skin.
Request for a quote
E-FILLERS.COM
Greece
HYAcorp Body Contouring MLF1© for body contouring is a resorbable high-purity skin implant-filler, which contains a pure viscous gel from cross-linked hyaluronic acid. That is a medical device for single use, designed specifically for giving the body contours, forming zones of buttocks, calves and correcting defects in the form of depressions.
Request for a quote
S.A.M MED BV
Netherlands
Syringe Testing is a critical step in developing and commercializing needlebased injection systems for medical use. By testing mechanical properties, such as breakloose and glide force, as well as biocompatibility, extractables and leachables, sterility, packaging and transit properties, we can determine if the device is within standard specifications, reducing the likelihood of improper function. Eurofins Medical Device Testing network of laboratories has experience performing an array of standard and nonstandard testing on syringes to verify their safety. Choose Eurofins Medical Device Testing to help you Determine the necessary testing requirements for your device Write GMPcompliant and ISO standard driven protocols and methods Perform the required tests to demonstrate that the expected performance criteria are satisfied Complete batch release activities and/or gain regulatory approval
Request for a quote
PIPELINEPHARMA
Lithuania
Simplify product discovery with the largest EU CTD dossier database. Expert curated, up-to-date information. Powerful search engine and product filters. Product categories include: generics, biosimilars, medical devices & nutraceuricals for human use. GMP approved factories. All therapy areas.
Request for a quote
OBSEQU GMBH
Germany
Class I of Regulation EU 2017/745 on medical devices; Type II R (liquidresistant), tested according to EN 14683 2019 for surgical masks. The inside and outside are made of physiologically harmless, plasticizerfree polypropylene fleece, the filter medium consists of a special microfiber fleece produced using the meltblown process, welded together using the ultrasound process.
Request for a quote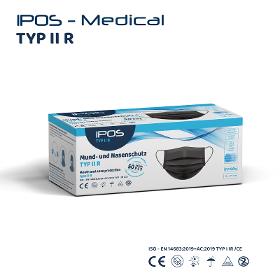
OBSEQU GMBH
Germany
Class I of Regulation EU 2017/745 on medical devices; Type II R (liquidresistant), tested according to EN 14683 2019 for surgical masks. The inside and outside are made of physiologically harmless, plasticizerfree polypropylene fleece, the filter medium consists of a special microfiber fleece produced using the meltblown process, welded together using the ultrasound process.
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
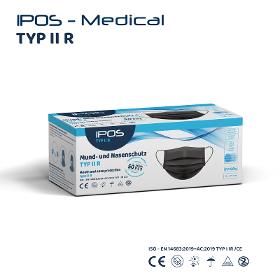
OBSEQU GMBH
Germany
Class I of Regulation EU 2017/745 on medical devices; Type II R (liquidresistant), tested according to EN 14683 2019 for surgical masks. The inside and outside are made of physiologically harmless, plasticizerfree polypropylene fleece, the filter medium consists of a special microfiber fleece produced using the meltblown process, welded together using the ultrasound process.
Request for a quote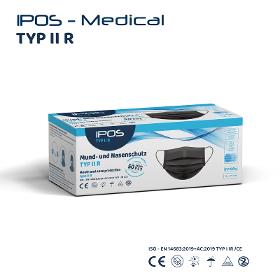
OBSEQU GMBH
Germany
Class I of Regulation EU 2017/745 on medical devices; Type II R (liquidresistant), tested according to EN 14683 2019 for surgical masks. The inside and outside are made of physiologically harmless, plasticizerfree polypropylene fleece, the filter medium consists of a special microfiber fleece produced using the meltblown process, welded together using the ultrasound process.
Request for a quote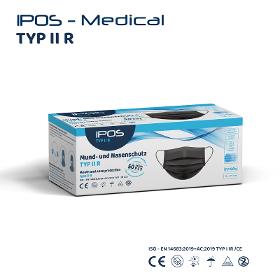
OBSEQU GMBH
Germany
Class I of Regulation EU 2017/745 on medical devices; Type II R (liquidresistant), tested according to EN 14683 2019 for surgical masks. The inside and outside are made of physiologically harmless, plasticizerfree polypropylene fleece, the filter medium consists of a special microfiber fleece produced using the meltblown process, welded together using the ultrasound process.
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Disinfectant solution for medical devices
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Concentrated disinfectant solution for medical devices
Request for a quoteILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Concentrated disinfectant solution for Medical Devices
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Disinfectant solution for medical devices 2 % Glutaraldehyde
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Concentrated disinfectant solution for medical devices Peracetic Acid 3840 %
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Disinfectant solution for medical devices Peracetic Acid 1,2 %
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Concentrated disinfectant solution for medical devices 2 % Gluteraldehyde
Request for a quote
NOBLE HEALTHCARE LTD
United Kingdom
A&D and Omron Blood Pressure Monitors, Digital Thermometers, Nebulisers, Wheeze Detector and Oximeter
Request for a quote
PSM VISION LIMITED
United Kingdom
Skincare, Haircare, Eyecare, Branded cosmetics (Eye, Lip, Face, Nail), Fragrances & Gift sets, Medical devices, Dental, Baby products, Vitamins & Health.
Request for a quoteResults for
Medical device - Import exportNumber of results
30 ProductsCountries
Company type
Category
- Medical Equipment (13)
- Medical fabrics (6)
- Beauty products (4)
- Sterilisation and disinfection - medical equipment (4)
- Aldehydes and derivatives (4)
- Disposable medical and surgical articles (2)
- Import-export - medical and surgical equipment (1)
- Skin-care products (1)
- Biomedical equipment (1)
- Hospital and medical services (1)
- Medical electronics - apparatus and equipment (1)
- Pharmaceutical products (1)
- Beauty creams (1)
- Prostheses, orthopedic (1)