- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- medical tests
Results for
Medical tests - Import export
S.A.M MED BV
Netherlands
Syringe Testing is a critical step in developing and commercializing needlebased injection systems for medical use. By testing mechanical properties, such as breakloose and glide force, as well as biocompatibility, extractables and leachables, sterility, packaging and transit properties, we can determine if the device is within standard specifications, reducing the likelihood of improper function. Eurofins Medical Device Testing network of laboratories has experience performing an array of standard and nonstandard testing on syringes to verify their safety. Choose Eurofins Medical Device Testing to help you Determine the necessary testing requirements for your device Write GMPcompliant and ISO standard driven protocols and methods Perform the required tests to demonstrate that the expected performance criteria are satisfied Complete batch release activities and/or gain regulatory approval
Request for a quote
BETTER AG
Switzerland
COVID-19 Test kit Testsealabs has developed the COVID-19 antigen test cassette 3in1 for quick detection. The COVID-19 Antigen Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of Covid-19 antigen in the nasopharynx, oropharynx and nasal swabs, to support the diagnosis of a Covid-19 virus infection. Technical specifications Testsealabs • 1 Box / 25 pack • Professional application (only for professional use) • Rapid test 3 in 1 • Approved in Germany • TÜV Süd EN ISO 13485:2016 • BFarm number: AT082 / 20 • CE certified and reimbursable test listed at the Federal Institute for Drugs and Medical Devices according to the Coronavirus Test Ordinance (TestV) • 97.6% sensitivity • 98.4% specificity • With a separate buffer solution
Request for a quote
BETTER AG
Switzerland
COVID-19 Test kit Greentspring has developed the COVID-19 antigen test cassette 4 in1 for quick detection. The COVID-19 Antigen Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of Covid-19 antigen in the lolli, nasopharynx, oropharynx and nasal swabs, to support the diagnosis of a Covid-19 virus infection. Technical specifications Greenspring • 1 Box / 25 pack • Professional application (only for professional use) • Rapid test 4 in 1 • Approved in Germany • EN ISO 13485:2016 • BFarm number: AT417/20 • CE certified and reimbursable test listed at the Federal Institute for Drugs and Medical Devices according to the Coronavirus Test Ordinance (TestV) • 96.77% sensitivity • 100% specificity • With a integrated buffer solution
Request for a quote
BETTER AG
Switzerland
COVID-19 Test kit Sejoy 5T has developed the COVID-19 antigen test cassette 3in1 for quick detection. The COVID-19 Antigen Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of Covid-19 antigen in the nasopharynx, oropharynx and nasal swabs, to support the diagnosis of a Covid-19 virus infection. CE certified (CE 1434) Sensitivity: 94,5 % Specificity: 99,9 % Clear result after less than 10 minutes Simple and painless application Fast test result CE certified and therefore approved throughout Europe CE 1434) BfArM listed evaluated by the Paul Ehrlich Institute reacts to the Omikron mutant very high reliability Recognizes a variety of mutations Set consisting of 1 test cassette 1 Sterile swab 1 extraction tube 1 extraction buffer 1 instruction leaflet 1 qualification certificate 1 workstation 1 biowaste bag Packing: 1 pcs.
Request for a quote
BETTER AG
Switzerland
COVID-19 Test kit Sejoy 5T has developed the COVID-19 antigen test cassette 3in1 for quick detection. The COVID-19 Antigen Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of Covid-19 antigen in the nasopharynx, oropharynx and nasal swabs, to support the diagnosis of a Covid-19 virus infection. CE certified (CE 1434) Sensitivity: 94,5 % Specificity: 99,9 % Clear result after less than 10 minutes Simple and painless application Fast test result CE certified and therefore approved throughout Europe CE 1434) BfArM listed evaluated by the Paul Ehrlich Institute reacts to the Omikron mutant very high reliability Recognizes a variety of mutations Set consisting of 1 test cassette 1 Sterile swab 1 extraction tube 1 extraction buffer 1 instruction leaflet 1 qualification certificate 1 workstation 1 biowaste bag Packing: 5 pcs.
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed

S.A.M MED BV
Netherlands
Ce système tout-en-un sans bandelettes dispose de 50 tests sur un ruban continu et d'un autopiqueur intégré contenant une cartouche de 6 lancettes. Avec ce système unique il ne faut plus s’inquiéter de manquer de bandelettes ou de lancettes. Des mesures faciles et rapides en seulement 4 étapes simples.
Request for a quote
S.A.M MED BV
Netherlands
The Lyme IgG/IgM Rapid Test Cassette is a qualitative test kit. Detects IgG and IgM antibodies to Borrelia in whole blood, serum or plasma specimens. This test consists of two components, an IgG component and an IgM component. GP Medical Professional Testing Kits CE Marked for Professional Use
Request for a quote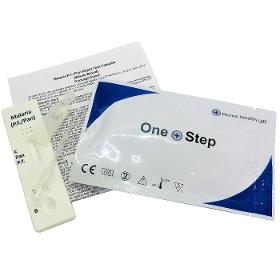
S.A.M MED BV
Netherlands
Diagnostic Automation offers a One-Step Malaria Rapid diagnostic Test for Malaria pf and pv antigens. This Malaria Rapid Test is a rapid, qualitative test for the detection of Plasmodium falciparum and/or Plasmodium vivax in whole blood. For the rapid qualitative determination of Malaria P. falciparum pecific histidine rich protein-2 (Pf HRP-2) and Malaria P. vivax specific lactate dehydrogenase (pvLDH) in human blood as an aid in the diagnosis of Malaria infection. Diagnostic Automation offers two formats of the Malaria Rapid Test.
Request for a quote
S.A.M MED BV
Netherlands
Diagnostic Automation offers a One-Step Malaria Rapid diagnostic Test for Malaria pf and pv antigens. This Malaria Rapid Test is a rapid, qualitative test for the detection of Plasmodium falciparum and/or Plasmodium vivax in whole blood. For the rapid qualitative determination of Malaria P. falciparum pecific histidine rich protein-2 (Pf HRP-2) and Malaria P. vivax specific lactate dehydrogenase (pvLDH) in human blood as an aid in the diagnosis of Malaria infection. Diagnostic Automation offers two formats of the Malaria Rapid Test.
Request for a quote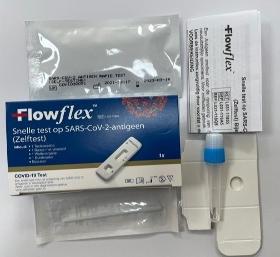
S.A.M MED BV
Netherlands
The FlowFlex Rapid Lateral Flow Antigen Test Kit uses the latest patented technology to detect proteins, ensuring that it can detect new strains of the COVID-19 virus. Using a simple nasal swab within 2cm of the nose makes it extremely easy to administer an accurate test.
Request for a quote
ISTANBUL PHARMA
Turkey
Our company is serving generally as medication and medical material wholesaler and distributor. It has a wide range of products including medication and medical devices and consumables. Keeps the health care market alive by worldwide service and provides attendance of new participants. Lends a hand to every institution that need help about medication and medical materials. The main purpose of this company is to support human health care. Incorporates many products like mask, covid-19 products, gloves, overalls, consumables, orphan medications, medical equipment’s, test kits, medical devices and disinfectants. Stores and distributes them very carefully. Keeps its studies without discriminating geographic location during supplying necessary materials and supports every region. Besides, our logistically strong company, directly refers necessary consumables to the required area. In natural disasters like earthquakes, consumables and medications are sent with health care officers.
Request for a quote
ALFA-MED LLC
Russia
Sensitiv imago® 530 is the previous model in the SensitivE line. Hi-tech hardware-software complexes for diagnostic scan, analysis and healing of organism. Senstiv imago 530 device is being assembled according to the new European quality management standards 9001 full accepted in EU in 2013. The device passed the medical laboratory testing class 2, according to the European Directive.
Request for a quoteResults for
Medical tests - Import exportNumber of results
13 ProductsCountries
Company type