- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- medical tests
Results for
Medical tests - Import export

EC PLAZA
South Korea
Our company has been growing for 20 years with the goal of One Health. - Mission: Contribute to human and animal health with diagnostic business. - Vision: Company supplying the world’s highest quality diagnostic kits.
Request for a quote
S.A.M MED BV
Netherlands
Syringe Testing is a critical step in developing and commercializing needlebased injection systems for medical use. By testing mechanical properties, such as breakloose and glide force, as well as biocompatibility, extractables and leachables, sterility, packaging and transit properties, we can determine if the device is within standard specifications, reducing the likelihood of improper function. Eurofins Medical Device Testing network of laboratories has experience performing an array of standard and nonstandard testing on syringes to verify their safety. Choose Eurofins Medical Device Testing to help you Determine the necessary testing requirements for your device Write GMPcompliant and ISO standard driven protocols and methods Perform the required tests to demonstrate that the expected performance criteria are satisfied Complete batch release activities and/or gain regulatory approval
Request for a quote
CRANAGE EMC & SAFETY
United Kingdom
Medical device testing is tightly regulated due to the potential severity of an EMC or Safety failure. The term 'medical device' covers a wide range of items and we have extensive experience in testing in this area, covering items such as prosthetic limbs, hospital beds, patient monitors, TENS machines, blood pressure monitors, to name just a few. We are UKAS accredited to test for the Medical standards IEC/EN 60601-1 and IEC/EN 60601-1-2, covering the basic safety and essential performance of medical electrical equipment. We are also UKAS accredited for the following specific medical standards: -IEC 60601-1-11 Particular requirements for medical electrical equipment and medical electrical systems in the home healthcare environment -IEC 60601-2-10 Particular requirements for the safety of nerve and muscle stimulators Using a UKAS accredited laboratory gives manufacturers assurance accuracy, reliability and consistency.
Request for a quote
CENTER OF INFORMATION TECHNOLOGIES NELIAN LLC
Russia
Dianel®-Micro Microscopy Analysis Software for Health Hiagnostic with digital dark-field microscope or bright- field microscope supports the following Blood analysis methods: LBA (Live Blood Analysis, Hemaview) Dark-field blood microscopy according to Enderlein Blood analysis by Bradford system called HLB Dry blood analysis Oxidative stress levels and vitamin deficiencies (by Lagard and Heitan) Inflammations observable under the microscope Pleomorphism study by Bechamp microzymas Blood and tissues analysis by Dr. Prof. Gunther Enderlein concept of the pleomorphism of microorganisms Blood and tissues analysis by Dr. Prof. Gunther Enderlein hypotheses about the origins of cancer Classic medical Clinical Complete Blood Count research Research on diseases of saliva Assessment of the condition of the skin, nails and hair in cosmetology Free two-mounts trial available Free support and software updates
Request for a quote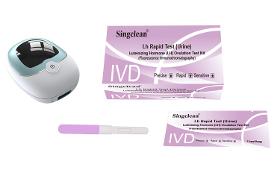
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
This kit is used for the quantitative determination of Luteinizing Hormone (LH) in human urine samples in vitro. Human luteinizing hormone (LH) is a kind of glycoprotein hormones secreted by the pituitary gland, exists in human blood and urine, its role is to stimulate the release of the ovaries mature eggs. LH in the middle of the menstrual secretion, sharply form LH peak, from the basic level of 5 ~ 20 miu/ml rapidly rising to the peak of 25 ~ 200 miu/ml.Urine LH concentration is usually about 36 ~ 48 hours before ovulation suddenly jumped sharply, in 14 ~ 28 hours peak, peak about 14 to 28 hours after theca burst, mature eggs. LH within two days after ovulation back to the basic level, to enter the luteal phase, luteal phase usually lasts for 14 days. Then, the new follicles began to development, into the next menstrual cycle, so the cycle, until the pregnancy. Women in the LH peak within 1 ~ 3 days after the most fertile.
Request for a quote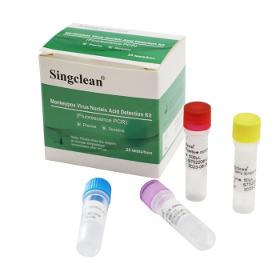
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
Monkeypox Virus Nucleic Acid Detection Kit (Fluorescence PCR) is used for the detection of nucleic acid from monkeypox virus in serum and human pustular or vesicular rash specimens from individuals suspected of monkeypox virus infectious. By specifically detecting the nucleic acid fragments of monkeypox virus, monkeypox virus can be quickly identified, which is suitable for rapid diagnosis of related diseases caused by monkeypox virus infection.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
[Intended use] HBsAg Test kit (Colloidal Gold) is used for qualitative detection of Hepatitis B surface Antigen in human serum or plasma samples. Hepatitis B surface Antigen (HBsAg) is the hepatitis b virus coat protein. It's not contagious, only the antigenicity. But it is usually accompanied by the presence of hepatitis b virus, so it is a sign of the hepatitis b virus infection. [Test principle] HBsAg Test kit (Colloidal Gold) uses colloidal gold immune chromatography technology to qualitative detection of Hepatitis B surface Antigen in human serum or plasma samples.
Request for a quote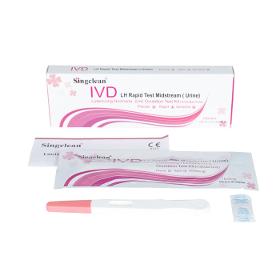
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
it is intend for qualitative determination of LH levels in women urine to predict ovulation time, so as to guide reproductive women to choose the optimal conception time or safe period contraception. Luteinizing Hormone(LH)Ovulation Test Kit based on the principle of colloidal gold labeling technique and immune chromatography of double-antibody sandwich method. During testing , the LH in urine specimen migrates via capillary action along the membrane to react with LH monoclonal antibody conjugated with nanoscale colloidal gold particles forming an antibody-antigen complex.
Request for a quote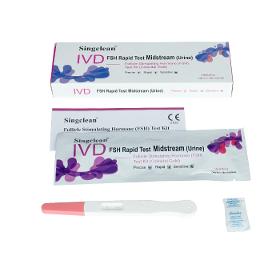
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
It is intend for qualitative determination of FSH levels in women urine. It provides some useful information for women who will prepare for pregnancy or focus on ovarian health, functions and fertility, also assists the diagnosis and treatment of pituitary and gonadal dysfunction. Follicle Stimulating Hormone(FSH) Test Kit (Colloidal Gold) is based on the principle of colloidal gold labeling technique and immune chromatography of double-antibody sandwich method. During testing, the FSH in urine specimen migrates via capillary action along the membrane to react with FSH monoclonal antibody conjugated with nanoscale colloidal gold particles forming an antibody-antigen complex.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
The human chorionic gonadotropin (hCG) pregnancy test kit is a rapid, one-step lateral flow immunoassay for the qualitative delection of human chorionic gonadotropin (hCG) in urine to aid in the detection of pregnancy. The test utilizes a combination of antibodies including a monoclonal hCG antibody to selectively detect elevated levels of hCG in urine to aid in the early detection of pregnancy for self-testing. It obtained the result from the colored lines.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
[Product Name] Generic Name: Alpha-fetoprotein (AFP) assay kit (ELISA) [Pack size]: 48 tests / kit, 96 tests / kit [Intended Use]: The Alpha-fetoprotein (AFP) assay kit is intended for quantitative determination of the Alpha-fetoprotein (AFP) concentration in human serum. [Introduction]: Alpha-fetoprotein (AFP) is a kind of embryonal single polypeptide which synthesized by embryo liver, yolk sac and gastrointestinal tract. Human AFP belongs to the alpha globulin, composed of 18 kinds of amino acids. The molecular weight of AFP is about 64 KD ~ 72 KD with a half-life of about 5d. Part of AFP can enter into the maternal blood circulation as amniotic fluid, AFP levels in body fluids of pregnant women all have a different enhanced. Normally, non-pregnancy women have lower concentration of serum AFP. Liver cancer is one of common reasons that induce abnormally increase of AFP.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
[Product name] Bacterial Vaginosis Quick Test Kit (Sialidase Detection) [Packing size] 20 pcs/kit; 50 pcs/kit [Intended use] For qualitatively testing activity of neuraminidase in female’s vaginal fluid, only for auxiliary diagnosis of bacterial vaginosis. [Testing principle] Neuraminidase is one of pathogenic factors of bacterial vaginosis (BV). Activity of neuraminidase in vaginal fluid of BV women is significantly higher than that in vaginal fluid of normal women; there is no or only a small amount of neuraminidase in vaginal fluid of normal women. By testing for neuraminidase higher than normal level in the vaginal fluid, patients can be diagnosed whether have BV. This kit mainly contains bacterial neuraminidase-specific chromogenic substrate solution.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
The test kit is used for the quantitative determination of procalcitonin (PCT) in human serum, plasma or whole blood samples in vitro. Procalcitonin (PCT) is the prohormone of calcitonin (CT), which is generally less than 0.1 ng/mL in the blood of healthy people, and its secretion increases after being stimulated by pro-inflammatory responses, especially after bacterial infection. Procalcitonin is an important marker that can specifically distinguish bacterial infection from inflammatory response caused by other reasons. Viral infection, allergy, autoimmune disease and transplant rejection do not cause significant elevation of procalcitonin, while local bacterial infection can result in moderate elevation of procalcitonin concentrations. In some cases (neonatal, multiple trauma, burns, major surgery, prolonged or severe cardiogenic shock), the elevation of procalcitonin may not be related to infection and usually returns to normal values quickly.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
[Product name] Interleukin-6(IL-6)Test Kit (Fluorescence immunochromatography) [Packaging specifications] 10 tests/box, 20 tests/box, 25 tests/box, 30 tests/box, 50 tests/box, 100 tests/box. [Indicated use] The kit is used for quantitative determination of interleukin-6 (IL-6) in human serum, plasma or whole blood samples in vitro. Interleukin-6 is mainly produced by Th2 cells and secreted by T lymphocytes, B lymphocytes, fibroblasts, endothelial cells, keratinocytes, hepatocytes and bone marrow cells. Its relative molecular weight is 26kDa. It is a glycoprotein composed of 212 amino acid residues. Interleukin-6 is the first biomarker to appear after inflammatory reaction. In different inflammatory diseases, the content of interleukin-6 is significantly different
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
[Product name] Creatine Kinase Isoenzyme MB (CK-MB) Test Kit (Fluorescence Immunochromatography) [Packaging specifications] 10 tests/box, 20 tests/box, 25 tests/box, 30 tests/box, 50 tests/box, 100 tests/box. [Intended use] This kit is used for the quantitative determination of Creatine Kinase Isoenzyme MB (CK-MB) in human serum, plasma or whole blood samples in vitro. There are four isozyme forms of Creatine Kinase (CK): muscle type (MM), brain type (BB), mixed type (MB) and mitochondrial type (MiMi), among which MB type is mainly present in myocardial cells. In myocardial infarction, creatine kinase increases within 6 hours of onset, peaks at 24 hours, and returns to normal within 3~4 days. Among them, Creatine Kinase Isoenzyme MB MB has high diagnostic specificity, so it has become one of the markers of myocardial infarction.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
[Product Name] HIV-1/2 antibody urine test kit (Colloidal Gold Method) [Packaging Specifications] Cassette: 1 test/box, 10 tests/box, 20 tests/box, 50 tests/box. [Intended Use] his product is used for the qualitative determination of human immunodeficiency virus type I (HIV-1) antibodies and type II (HIV-2) antibodies in human urine samples. It is suitable for the auxiliary diagnosis of HIV infection. The test results are only for clinical reference and cannot be used alone as the basis for confirming or excluding cases. In order to achieve the purpose of diagnosis, the test results should be used in conjunction with clinical examination, medical history and other examinations. This product can be used for consumer self-test.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
IIntended Use Singclean Flu A/B Antigen Test Kit is an in vitro qualitative detection that uses colloidal goldimmunochromatography to test influenza A/B antigen in nasopharyngeal swab samples obtained from patients with signs and symptoms of respiratory infection in just 15 minutes. lt is designed to give rapid and reliable test results on the spot without any laboratory equipment. This allows early start of treatment,which helps reduce the duration and severity of the disease,Besides,detection of influenza reduces unnecessary use of antibiotics and aids in limiting thespread of the infection. Advantages a. Easy to operate: All components include in kit,no additional equipment orlaboratory resources required; b.Fast detection: 15 minutes to interpret the result after collecting specimen; c.Joint testing: Influenza A and B can both be tested in one reagent; d.Accurate,Rapid,Sensitive Positive: Positive influenza A and B:Line C, Line A and Line B allappeared.
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
The novel coronaviruses belong to the βgenus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection: asymptomatic only infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
Singclean Multi-Drug One Step Test Kit is used to qualitatively detectwhether there are drugs or drug metabolites in the urine of targetpeople,and conduct preliminary screening for target drug users withcolloidal gold method. lt is for the detection of 23 drugs like COC、AMP、MOP、THC、MET、OPI、BAR、BZO、MTD、MDMA、TCA、OXY、BUP、PCP、EDDP. ldeal for: 1.Pre-employment drug screening 2.Random drug testing 3.Medical clinic drug testing 4.Reasonable suspicion drug tests 5.Substance abuse screening in hospitals How much sample do I need? 1. Cassette format: need 3 drops of urine sample(about 100uL). 2. Panel format: lmmerse the test panel verticallyinto the urine specimen for at least 10-15 secondsor until migration occurs.mmerse the strip(s) atleast the level of the wavy lines, but not above thearrow(s) on the test card. 3.Cup format: fill the Drug Test Cup with urine to at least the minimum fill level line. POSITIVE Only one colored band appears in the control region (C)
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
Microalbuminuria (MAU) Rapid Test Kit (Colloidal Gold) is intended for qualitative determination of albuminuria levels in urine in vitro diagnostic. It is used as a supplementary method in the diagnosis of chronic kidney injury(CKI). Microalbuminuria(MAU) is a term to describe a moderate increase in the level of urine albumin. It is also named urine microalbuminuria(UMA or mALB) Albumin is a normal protein in the blood, But under physiological condition sit appear only very small amounts of albumin in the urine
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
Monkeypox virus Antigen Test Kit (Immunochromatography) is a solid phase immunochromatographic assay for the rapid, qualitative detection of antigen to Monkeypox virus in human body fluid. This test provides only a preliminary test result. Therefore, any reactive specimen with the Monkeypox virus Antigen Test Kit (Immunochromatography)must be confirmed with alternative testing method(s) and clinical findings.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
The novel coronaviruses belong to the βgenus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection: asymptomatic only infected people can also be an infectious source. Based on the current epidemiological investigation, the incubation period is 1 to 14 days, mostly 3 to 7 days. The main manifestations include fever, fatigue and dry cough. Nasal congestion, runny nose, sore throat, myalgia and diarrhea are found in a few cases.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
INTENDED USE COVID-19 IgG/IgM Test kit(Colloidal Gold Method)is a solid phase immunochromatographic assay for the rapid, qualitative and differential detection of IgG and IgM antibodies to Novel Coronavirusi in human whole blood, serum or plasma. This test provides only a preliminary test result. Therefore, any reactive specimen with the COVID-19 IgG/IgM Test kit(Colloidal Gold Method) must be confirmed with alternative testing method(s) and clinical findings. PACK FORMATS 1 test/box ,20 test/box,50 test/box ,100 test/box INTRODUCTION The novel coronaviruses belong to the β genus. COVID-19 is an acute respiratory infectious disease. People are generally susceptible. Currently, the patients infected by the novel coronavirus are the main source of infection; asymptomatic infected people can also be an infectious source.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
Intended Use COVID-19 Test Kit (Colloidal Gold Method) is a solid phase immunochromatographic assay for the rapid, qualitativedetection of antigen to 2019 Novel Coronavirus in human nasal cavity. This test provides only a preliminary result forself-testing. Therefore, any reactive specimen with the COVID-19 Test Kit (Colloidal Gold Method) must be confirmedwith alternative testing method(s) and clinical findings. Features ·For self-testing use ·Results ready in 15 minutes ·Accurate diagnostic tool for active infection·Easy to administer and read results ·Affordable, no need for instrument, highly portable ·Enable testing on a massive scale COVID-19 Positive: lf the C and T band is present, the test indcates for the presence of COVID-19 (SARS-Cov-2) antigen in the specimen.The result is COVID-19 positive.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
Monkeypox virus IgG/IgM Test Kit (Immunochromatography) is a solid phase immunochromatographic assay for the rapid, qualitative detection of IgG/IgM antibody to Monkeypox virus in human blood. This test provides only a preliminary test result. Therefore, any reactive specimen with the Monkeypox virus IgG/IgM Test Kit (Immunochromatography) must be confirmed with alternative testing method(s) and clinical findings.
Request for a quote
HANGZHOU SINGCLEAN MEDICAL PRODUCTS
China
NGAL Rapid Test Kit applies colloidal gold lmmunol Chromatography, for the qualitative detection of NeutrophilsGelatinase Associated Lipocalin (NGAL) in urine. The test is used as an aid in the early diagnosis of acute kidneyinjury,risk classification and treatment monitoring. Acute kidney injury (AKI) is where your kidneys suddenly stop working properly. It can range from minor loss ofkidney function to complete kidney failure. Symptoms of acute kidney injury: feeling sick or being sick diarrhoea dehydration peeing less than usual confusion drowsiness It's essential that AKl is detected early and treated promptly.lf the kidneys shut down completely, this may require temporary support from a dialysis machine, or lead to death. Positive: Two colored lines are visible
Request for a quote
BETTER AG
Switzerland
COVID-19 Test kit Greentspring has developed the COVID-19 antigen test cassette 4 in1 for quick detection. The COVID-19 Antigen Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of Covid-19 antigen in the lolli, nasopharynx, oropharynx and nasal swabs, to support the diagnosis of a Covid-19 virus infection. Technical specifications Greenspring • 1 Box / 25 pack • Professional application (only for professional use) • Rapid test 4 in 1 • Approved in Germany • EN ISO 13485:2016 • BFarm number: AT417/20 • CE certified and reimbursable test listed at the Federal Institute for Drugs and Medical Devices according to the Coronavirus Test Ordinance (TestV) • 96.77% sensitivity • 100% specificity • With a integrated buffer solution
Request for a quote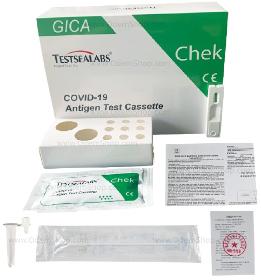
BETTER AG
Switzerland
COVID-19 Test kit Testsealabs has developed the COVID-19 antigen test cassette 3in1 for quick detection. The COVID-19 Antigen Rapid Test Cassette is a rapid chromatographic immunoassay for the qualitative detection of Covid-19 antigen in the nasopharynx, oropharynx and nasal swabs, to support the diagnosis of a Covid-19 virus infection. Technical specifications Testsealabs • 1 Box / 25 pack • Professional application (only for professional use) • Rapid test 3 in 1 • Approved in Germany • TÜV Süd EN ISO 13485:2016 • BFarm number: AT082 / 20 • CE certified and reimbursable test listed at the Federal Institute for Drugs and Medical Devices according to the Coronavirus Test Ordinance (TestV) • 97.6% sensitivity • 98.4% specificity • With a separate buffer solution
Request for a quote
BODYVIE
United Kingdom
At Bodyvie, we offer a wide range of blood tests which are then processed via The Doctors Laboratory, a world-leading diagnostics laboratory. Turnaround time on blood results are quick with many being available in 4 hours. This valuable information helps evaluate your health and aid in general health management.
Results for
Medical tests - Import exportNumber of results
79 ProductsCountries
Company type
Category
- Medical tests (42)
- Medical Equipment (16)
- Connectors, electronic (8)
- Pharmaceutical products (4)
- Electric cables (2)
- Biomedical equipment (1)
- CAD/CAM Computer Assisted Design/Computer Assisted Manufacturing - software (1)
- Chemicals - Basic Products & Derivatives (1)
- Diagnosis reagents (1)
- Digital displays - indication tubes (1)
- Electronic components (1)
- Gloves (1)
- Hospital and medical services (1)
- Industrial microbiology (1)
- Lasers - medical applications (1)
- Magnetic equipment (1)
- Medical electronics - apparatus and equipment (1)
- Medical services - specialised (1)
- Non-destructive tests (1)
- Paints, synthetic resin (1)