- europages
- >
- Medical Equipment
- >
- TULPAR HEALTH PRODUCTS
TULPAR HEALTH PRODUCTS
Turkey
Manufacturer/ Producer

TULPAR HEALTH PRODUCTS
Turkey
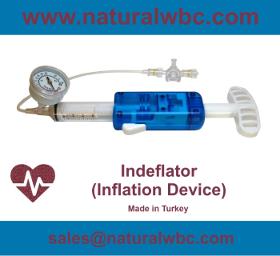
Ergonomic design Positive and negative pressure control Utmost precision 30 Bar (435 PSI) pressure resistant 20 / 30 ml Volume options ISO13485:2016 ISO9001:2015 Medical Devices Directive (93/42/EEC)
TULPAR HEALTH PRODUCTS
Turkey
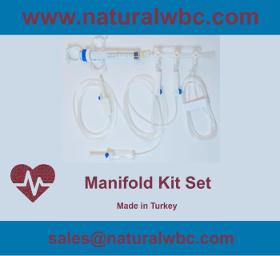
3 way manifold 1 pc Control syringe 12cc 1 pc Infusion set with air-vent 1 pc Infusion set without air-vent 1 pc M/F Pressure Line Manifold 3 way 500 PSI Pressure resistant Transparent body Large inner lumen Luer connector with swivel head Control Syringe 12 cc Ergonomic design Lock & control mechanism Luer connector Transparent body Air-leak preventing extended reservoir Right-on, right-off flexible pivoting latches Infusion Set With / Without Air-Vent Spike (ABS) or air outlet (with protective cover) Drip Chamber with or without Liquid Filter (PVC)(15 μm liquid filter nominal pore size) Flow regulator Injection site (Natural latex / latex free, Y-type injection) Luer slip or luer lock connection 150 cm Pressure Line 250 PSI Pressure resistant extension Line 3.0 x 4.1mm hose Luer lock connector Rotating, push-pull, click-action model Transparent body ISO13485:2016 ISO9001:2015 Medical Devices Directive (93/42/EEC)
TULPAR HEALTH PRODUCTS
Turkey
- FFP2 NR - 4-Layer Filter Protection - PFE ≥ %95 - Easy-use 3D Structure - 3-Panel Protective Respirator - Anti-Fog Feature around Nose Line - Spandex Ear Strap with Comfortable Fit - No Pain on Ears - No Scratch on Nose - Nose Support Strip - Comfortable Aspiration - Individual Package - Size: 210mm x 175mm ±10 - EN149 standard, with NB 2163 CE - Daily Output: 100.000 - Made in Turkey EN149 standard, with NB 2163 CE Daily Output: 100.000 Made in Turkey
Tulpar Health Products; committed to its mission, vision and core values; all over the world particularly those that have taken place in the medical sector in Turkey and the Middle East and European countries; is a manufacturing and sales company predominantly in the field of interventional cardiology. Our products in the field of Interventional Radiology, which we are proud of them, are first in Turkey. Our company continues to be a solution partner with our endless energy in the production of specific and new products. Our company has the ISO13485: 2016, ISO9001: 2015, Medical Device Directive 93/42/EEC quality certificates required for medical and health products to take their place in the world markets. Certifed Products We Produce: Indeflator Manifold Kit Set Other Interventional Cardiology Medical Equipments
Website
Manufacturer/ Producer
30 Agustos Mh. Sehit Hasan Oztürk Cad. No:7/5 Etimesgut
06794 Ankara - Turkey
Company info
Key figures
-
- Company headcount
- 11 – 50
-
- Sales staff
- 11 – 50
-
- % of export sales
- 20%
Organisation
-
- Year established
- 2017
-
- pages.epage-home.site-status
- Registered office
-
- Main activity
- Manufacturer/ Producer
Activities of TULPAR HEALTH PRODUCTS
- Medical Equipment
- Interventional Cardiology
- angioplasty equipment
- certified medical equipment
- Medical equipment manufacturer
europages also recommends
A selection of companies related to the activity:
A selection of products that might interest you

ANSMANN AG
Germany
The new IPC charger series is the latest innovation in the field of quick-charging. The universal chargers have integrated communication and support all popular communication protocols, such as CAN, I2C, SM, LIN, HDQ. Li-ion rechargeable battery packs from 24 V to 48 V will be automatically detected. Once the E-BUS charger has made contact with the rechargeable battery, an extensive diagnostics procedure is started. The intelligent chargers identify the rechargeable battery voltage, the charge current is adapted and the charge process started. The output power here is up to 300 W. This enables the shortest possible charge times with simultaneous exchange of parameters to be realised with applications such as in drive systems, power tools or medical equipment. With the IPC 300W we are delighted to present a universal charger that can adapt to all lithium ion rechargeable battery systems.
Request for a quote
ANSMANN AG
Germany
Planning a new development or a redesign of a rechargeable battery or charger? ANSMANN – Your partner from the initial idea through the planning, design, development and manufacturing stages and on to serial manufacturing. Based on your application and the requirements, we can guide you with professional advice for the selection of the correct cells, the capabilities of the necessary protective circuitry, compliance with standards and certification (as early as in the development phase), the first prototypes as STL parts and the first measurements. We can support you through all of the important steps towards your final decision. We can take on the design and manufacturing of the housing parts and the injection and stamping tools required for this. Single-source delivery and manufacturing guarantees optimum interaction between rechargeable battery, BMS and charger. The cells that we use are delivered by renowned manufacturers such as Panasonic, LG, Samsung, Sanyo, A123, Sony, etc.
Request for a quote
SOL-MILLENNIUM
Poland
Non - sterile single use polypropylene holder for safe and effective blood collection.
Request for a quote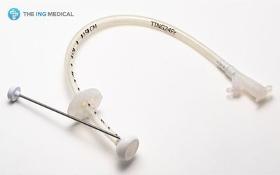
EC PLAZA
South Korea
1) Product Description The Gastrostomy Feeding Tube are indicated for use in patients who require long term feeding, are unable to tolerate oral feeding, who are at low risk for aspration, require gastric decomparession. It is a tube used to supply food and drugs to the stomach. 2) Product Features - Easy and less traumatic to insert due to the soft and compressible internal dome. - Radiopaque dome allows x-ray placement confirmation - Identification of tube obstructions enhanced with translucent tubings - Soft, less traumatic silicone designed for patient comfort
Request for a quoteRequest for quotes
Create one request and get multiple quotes form verified suppliers.
- Only relevant suppliers
- Data privacy compliant
- 100% free