- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- medical device
Results for
Medical device - Import export
TEMAS GROUP EXPORT PARTNERS
Turkey
MUSTANOGLU PROFESSIONAL MEDICAL EQUIPMENTS SALON DEVICESREGIONAL REVIEW REGIONAL THINNING DIODE LASER EMS SLIM EMS HIEMT 7D HIFU 4D HIFU HYDRAPRO Q-SWITCH PLASMA G5 LENF DRAINAGE INBODY PDT SKIN ANALYSIS ROLL SLIM
Request for a quote
MMAYR TRADING CO, LTD
Viet Nam
We can be proud to work with the most qualified glove manufacturer in Vietnam. Sourcing nitrile gloves has become a business with a lot of fraud and you have to be very careful. that’s why we are even happier to have the best factory in Vietnam on our side. Our documentations and certificates are the most qualified papers you can get from a gloves manufacturer in Vietnam right now. We look forward to supplying you with our excellent products and service. We are a top level clinical and medical device company that manages everything from A to Z, from manufacturing to logistics. this is precisely what separates us from ‘the pack. We offer oxygen concentrators, medical gloves, oximeters, face shields, all type of masks,oxygen bottles and all type off masks, sanitiecers, Air cleaning purifieres-saniticers
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Disinfectant solution for medical devices
Request for a quote
ALFA-MED LLC
Russia
The work of the TempoHeart module is based on the method of scoring the heart rate vegetative regulation. This method is popular due to its high information value and non-invasiveness. Cannot be used without an interface block. The module can: • evaluate the heart rate autonomic regulation; • to predict in advance (to prematurely reveal) the hazards of a myocardial infarction, hypertensive crises and strokes; • to determine the metabolic and electrolyte disorders in the myocardium; • identify risk groups for heart rate increased stability; • to make a cold evaluation of the autonomic nervous system's reaction when exposed to electromagnetic fields, intoxications and other pathogenic factors; • to assess the activity of the respiratory component when measuring heart rate variability, recording the parameters of blood movement in the vessels; • to expose the stress resistance level.
Request for a quote
TEDCAS S.L.
Spain
This innovative solution enables an immersive remote connection experience with operating rooms to improve the quality of care for technicians and surgeons. Main Features: VIDEOCONFERENCE Allows communication with technicians or colleagues who are required during the intervention. REMOTE CONTROL Allows remote control of medical equipment in the operating room to make support more efficient. It can be several equipments simultaneously and multi-brand. VIDEO CAPTURE Connect as many capturers as you wish and share the image of the operating room screens with other colleagues. Our software will allow you to generate pre-formatted layouts with the images. CAMERA CONTROL A PTZ camera is included in the operating room, which can be remotely controlled through the application, in order to explore the environment in detail, rotating the camera and zooming in where needed.
Request for a quote
TEDCAS S.L.
Spain
Let virtual and mixed reality transform your preoperative training in surgical planning and skills. PRE-OPERATIONAL PLANNING: Get the complete image before entering the operating room. SURGICAL SKILLS TRAINING: Enhanced learning through virtual and mixed reality.
Request for a quote
TEDCAS S.L.
Spain
Make your presentation live and transform your audience engagement. ARP FEATURES: Transform your presentation with a wide range of tools. VIDEO CONFERENCE & LINK TO LIVE SURGERY: Be better connected with your audience.
Request for a quote
TEDCAS S.L.
Spain
This product allows to control, plug and play mode, any equipment into a surgery room. Just connect our receptor to the USB port and program your macros (mouse and keyboard movements). Assign a voice command to them and each you say it, the macro will be executed in the device.
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
Medical Device Services / Medical Device Laboratory Testing INVITRO-CONNECT GmbH offers you: - General regulatory advice for medical devices - All regulatory examinations and tests under GLP - Project management and study monitoring - Medical Device Regulatory Service - Product testing for contained SVHC substances INVITRO-CONNECT offers you a complete service for the approval of medical devices according (EU) 2017/745 (Medical Device Regulation - MDR) / ISO 10993. From the development of an ideal test strategy to laboratory and project coordination, study monitoring, toxicological expertise and GLP inspections in the cooperation laboratories. We deliver the finalized test reports including the registration dossier - digitally and on paper, worldwide. Benefit from our diverse know-how, our experienced cooperation partners and an international, very well-organized network. Questions? -> contact@invitro-connect.com
Request for a quote
CRANAGE EMC & SAFETY
United Kingdom
Medical device testing is tightly regulated due to the potential severity of an EMC or Safety failure. The term 'medical device' covers a wide range of items and we have extensive experience in testing in this area, covering items such as prosthetic limbs, hospital beds, patient monitors, TENS machines, blood pressure monitors, to name just a few. We are UKAS accredited to test for the Medical standards IEC/EN 60601-1 and IEC/EN 60601-1-2, covering the basic safety and essential performance of medical electrical equipment. We are also UKAS accredited for the following specific medical standards: -IEC 60601-1-11 Particular requirements for medical electrical equipment and medical electrical systems in the home healthcare environment -IEC 60601-2-10 Particular requirements for the safety of nerve and muscle stimulators Using a UKAS accredited laboratory gives manufacturers assurance accuracy, reliability and consistency.
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
Microbiological examinations / Microbiological tests against viruses, bacteriophages, bacteria, yeast, molds, mycobacteria and bacterial spores for disinfectants, medical devices, pharmaceutical products and cosmetics Our team of experts supports manufacturers of biocidal and medical products as well as cosmetics and pharmaceuticals scientifically in the context of approval projects. For example, we create safety assessments for cosmetics and biocompatibility assessments for medical devices. Here we focus in particular on infection prevention and wound treatment products. For more information please contact INVITRO-CONNECT GmbH for assistance in developing specialized protocols for your test materials. INVITRO-CONNECT GmbH: Fast Project Execution: personal - competent - reliable --> contact@invitro-connect.com
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
https://www.invitro-connect.com/en - Your International Experts for Laboratory Testing and Regulatory Services - More than 80 test laboratories in our network - Product safety - Regulatory service - in vitro tests - All OECD tests for pharmaceuticals, chemicals, medical devices and cosmetics - in-house toxicologistes - Project management - Study monitoring --> complete services - all from one hand !!! --> contact@invitro-connect.com We would like to manage also your projects. Best regards Pascal Piller and the whole team of INVITRO-CONNECT GmbH #biocides #toxicological #chemicals #laboratory #regulatory #affairs #reach #projectmanagement #pharma #medical #study #devices #Labor #GLP #cosmetics
Request for a quote
PIPELINEPHARMA
Lithuania
Simplify product discovery with the largest EU CTD dossier database. Expert curated, up-to-date information. Powerful search engine and product filters. Product categories include: generics, biosimilars, medical devices & nutraceuricals for human use. GMP approved factories. All therapy areas.
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Disinfectant solution for medical devices
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Concentrated disinfectant solution for medical devices
Request for a quoteILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Concentrated disinfectant solution for Medical Devices
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Disinfectant solution for medical devices 2 % Glutaraldehyde
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed

ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Concentrated disinfectant solution for medical devices Peracetic Acid 3840 %
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Disinfectant solution for medical devices Peracetic Acid 1,2 %
Request for a quote
ILLIYYUN SAV. OTO. DAN. LTD.
Turkey
Concentrated disinfectant solution for medical devices 2 % Gluteraldehyde
Request for a quote
PROEKT INJINIRING, LLC
Russia
A registration certificate is a document that allows you to legally convert a medical device (hereinafter referred to as a medical device, MI) on the territory of the Russian Federation. A registration certificate is issued on the basis of an Order of the registering authority (Federal Service for Supervision in the Field of Healthcare/Roszdravnadzor) after passing all the mandatory stages of state registration, during which the registration dossier is formed
Request for a quote
KWL EUROPEAN WAREHOUSING & LOGISTICS
Netherlands
Contact us for safe and secure European warehousing, fulfillment and distribution services for your medical, surgical, diagnostic and imaging devices and healthcare supplies. We are located in an important European Medtec area with various small, medium sized and big medical and pharmaceutical companies like Medtronic, Merit Medical, Abbott and Janssen Pharmaceutica. Since 2010 we already have various value added warehousing, logistic and distribution operations in place for various international medical device manufacturers and healthcare supplies distributors
Request for a quote
MAXCERT LTD
Germany
Maxcert is offering EC Representative services for non EU medical devices manufacturers. Maxcert is authorized/ registered EC REP service provider and can manage your ECREP requirements better than others. We make sure that you are MDR ready! and well updated with the EU regulations for your medical devices. Maxcert’s team has expert knowledge to help you navigate through the complex regulatory challenges that the new EU MDR bring. We can assist you through out the entire process to ensure that you and your business are compliant with all of the EU MDR requirements. For more information, reach out to us at
Request for a quote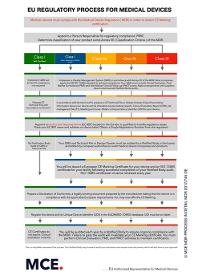
MAXCERT LTD
Germany
Our experienced team has for years done documentation for companies who are manufacturers of medical devices /surgical instruments. We provide Quality-Management-Systems for meeting legal and normative requirements. For manufacturers of medical devices / surgical instruments class I (including Is, Im, and new for MDR class Ir), IIa, and IIb we offer the following services: Implementation DIN EN ISO 13485 and the EU-Regulation 2017/745 MDR and 21 CFR 820 QSR Creating QM-Systems with procedural instructions and work instructions Technical Documentation Clinical evaluation Risk Management / Risk Analysis Essential Safety and Performance Requirements Validation of processes UDI-labeling Consulting for Regulatory Affairs Internal audits and external supplier audits Accompaniment and support with inspections by authorities and Audits by notified bodies Communication with authorities
Request for a quoteResults for
Medical device - Import exportNumber of results
25 ProductsCountries
Company type
Category
- Medical Equipment (15)
- Sterilisation and disinfection - medical equipment (4)
- Aldehydes and derivatives (4)
- Import-export - medical and surgical equipment (2)
- Scientific research centres and laboratories (2)
- Medical and surgical instruments (1)
- Disposable medical and surgical articles (1)
- Hospital and medical services (1)
- Pharmaceutical products (1)
- CAD/CAM Computer Assisted Design/Computer Assisted Manufacturing - software (1)
- Industrial microbiology (1)
- Medicine and surgery - apparatus and equipment (1)
- Quality - certification (1)
- Testing of products and materials (1)