- europages
- >
- COMPANIES - SUPPLIERS - SERVICE PROVIDERS
- >
- medical device
Results for
Medical device - Import export

INNOVATIVE SENSOR TECHNOLOGY IST AG
Switzerland
Mechanically robust and with a chemical and condensed waterproof sensing area, the digital IST AG HYT humidity modules offer a wide application window and an optimal price-performance ratio. Precisely calibrated, the HYT modules deliver an outstanding accuracy and excellent long-term stability even at high humidity - ideal for sophisticated mass applications, industrial handheld devices and precise humidity transmitters. Advantages of the HYT 221 digital humidity module include: — Calibrated and temperature compensated — Excellent humidity/temperature accuracy and stability — High chemical resistance — I2C protocol (address 0x28 or alternative address) — Wide humidity and temperature range (0 % RH to 100 % RH and -40 °C to +125 °C) — Very low drift — Very stable at high humidity — Interchangeable without adjustments
Request for a quote
MPS MICRO PRECISION SYSTEMS AG
Switzerland
Novostia, a Swiss startup founded in 2017, has entrusted MPS with the manufacture of its TRIFLO artificial valve, a revolutionary aortic prosthesis on several levels. Thanks to its aerodynamic design, the presence of three leaflets and the absence of physical pivot axes for the leaflets, the TRIFLO valve aims to satisfy the following three criteria: - A device lifespan at least equal to that of the patient, to avoid the need for a second surgical procedure. This means more than 30 million flap openings and closures per year, with no leaks, blockages, cracks, ruptures or flap escapes. - The artificial valve must not lead to thrombosis, so that patients do not need to take anticoagulants. - Operation (flap opening and closing, blood flow through the valve) must be as silent as possible, so as not to disturb the patient or those around him, day or night. The TRIFLO device is in clinical trial and is not approved for sale.
Request for a quote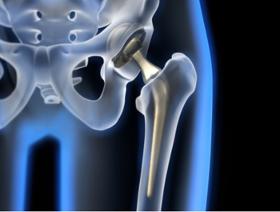
INVITRO-CONNECT GMBH
Switzerland
Medical Device Services / Medical Device Laboratory Testing INVITRO-CONNECT GmbH offers you: - General regulatory advice for medical devices - All regulatory examinations and tests under GLP - Project management and study monitoring - Medical Device Regulatory Service - Product testing for contained SVHC substances INVITRO-CONNECT offers you a complete service for the approval of medical devices according (EU) 2017/745 (Medical Device Regulation - MDR) / ISO 10993. From the development of an ideal test strategy to laboratory and project coordination, study monitoring, toxicological expertise and GLP inspections in the cooperation laboratories. We deliver the finalized test reports including the registration dossier - digitally and on paper, worldwide. Benefit from our diverse know-how, our experienced cooperation partners and an international, very well-organized network. Questions? -> contact@invitro-connect.com
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
Microbiological examinations / Microbiological tests against viruses, bacteriophages, bacteria, yeast, molds, mycobacteria and bacterial spores for disinfectants, medical devices, pharmaceutical products and cosmetics Our team of experts supports manufacturers of biocidal and medical products as well as cosmetics and pharmaceuticals scientifically in the context of approval projects. For example, we create safety assessments for cosmetics and biocompatibility assessments for medical devices. Here we focus in particular on infection prevention and wound treatment products. For more information please contact INVITRO-CONNECT GmbH for assistance in developing specialized protocols for your test materials. INVITRO-CONNECT GmbH: Fast Project Execution: personal - competent - reliable --> contact@invitro-connect.com
Request for a quote
INVITRO-CONNECT GMBH
Switzerland
https://www.invitro-connect.com/en - Your International Experts for Laboratory Testing and Regulatory Services - More than 80 test laboratories in our network - Product safety - Regulatory service - in vitro tests - All OECD tests for pharmaceuticals, chemicals, medical devices and cosmetics - in-house toxicologistes - Project management - Study monitoring --> complete services - all from one hand !!! --> contact@invitro-connect.com We would like to manage also your projects. Best regards Pascal Piller and the whole team of INVITRO-CONNECT GmbH #biocides #toxicological #chemicals #laboratory #regulatory #affairs #reach #projectmanagement #pharma #medical #study #devices #Labor #GLP #cosmetics
Request for a quoteDo you sell or make similar products?
Sign up to europages and have your products listed
Results for
Medical device - Import exportNumber of results
5 ProductsCompany type